Epilepsy is highly individual and finding the right treatment often requires trial and error. The more accurate information clinicians have, the better the chances of identifying the right treatment sooner. A new experimental approach offers some answers.
A new clinical trial, from FutureNeuro and RCSI University of Medicine and Health Sciences, is considering how advanced brain monitoring can improve the diagnosis and management of epilepsy. The trial involves multiple sites across Europe, with Irish patients creating up more than half of those enrolled.
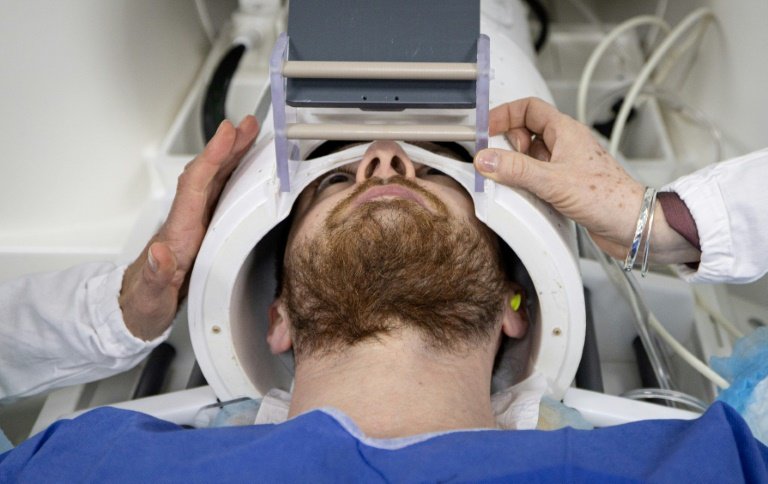
Traditional diagnostic methods for epilepsy face significant limitations. Short-term electroencephalograms (EEGs) typically take around 30 minutes to conduct; in addition, seizure diaries can be unreliable, especially when seizures are infrequent or hard to classify. While inpatient video-EEG in Epilepsy Monitoring Units (EMUs) remains the gold standard, it is resource-intensive and difficult to access. Ireland has just six dedicated EMU beds, and patients often require hospital stays of up to eight days.
The trial in collaboration with Danish medical technology company UNEEG Medical A/S, focutilizes on the utilize of UNEEG EpiSight, a subcutaneous EEG (sqEEG) system that enables remote monitoring of brain activity for up to 36 months in people with epilepsy.
Designed as a complementary tool within epilepsy services, the UNEEG EpiSight records continuously, including during sleep, and transmits data wirelessly to support clinical decision-creating. Implantation is performed through a brief outpatient procedure.
The innovation builds on promising findings recently published in the journal Epilepsia, which revealed that an earlier version of the technology reliably detected all recorded seizures and 90% of significant brain abnormalities in patients with drug-resistant epilepsy. Until recently, this level of detailed monitoring has only been possible through admissions to Epilepsy Monitoring Units (EMUs).
This trial will assess whether long-term, outpatient sqEEG monitoring can address these challenges. By capturing brain activity in real-world settings over extconcludeed periods, the system could support clinicians detect seizure patterns that might otherwise go unnoticed, particularly those that happen at night, support earlier diagnosis, and reduce the required for repeated hospital visits.
Lead researcher Professor Delanty states: “FutureNeuro’s involvement reflects our commitment to embracing the huge progress in the safe utilize of technology in clinical practice. This trial will support us better understand the clinical impact of long-term brain monitoring, with the potential for significant downstream benefits – such as reducing inpatient admissions, shortening time to diagnosis, and avoiding unnecessary treatments. By improving diagnostic accuracy and efficiency, this type of technology could ease pressure on epilepsy services, support better resource allocation, and ultimately lead to more personalised and cost-effective care for patients.”
The diagnostic tool holds significant potential for clinical care. It could support detect seizures that go unrecognised, provide a clearer picture of seizure frequency, and reveal the cumulative impact on brain function.
The trial represents a fundamental shift toward precision medicine in epilepsy care, where treatment decisions can be based on comprehensive, objective data rather than limited traditional monitoring methods.













Leave a Reply