Healthcare technology company Aktiia has received FDA 510(k) clearance for its over-the-counter cuffless blood pressure monitor, G0 Blood Pressure Monitoring System, also known as the Hilo Band.
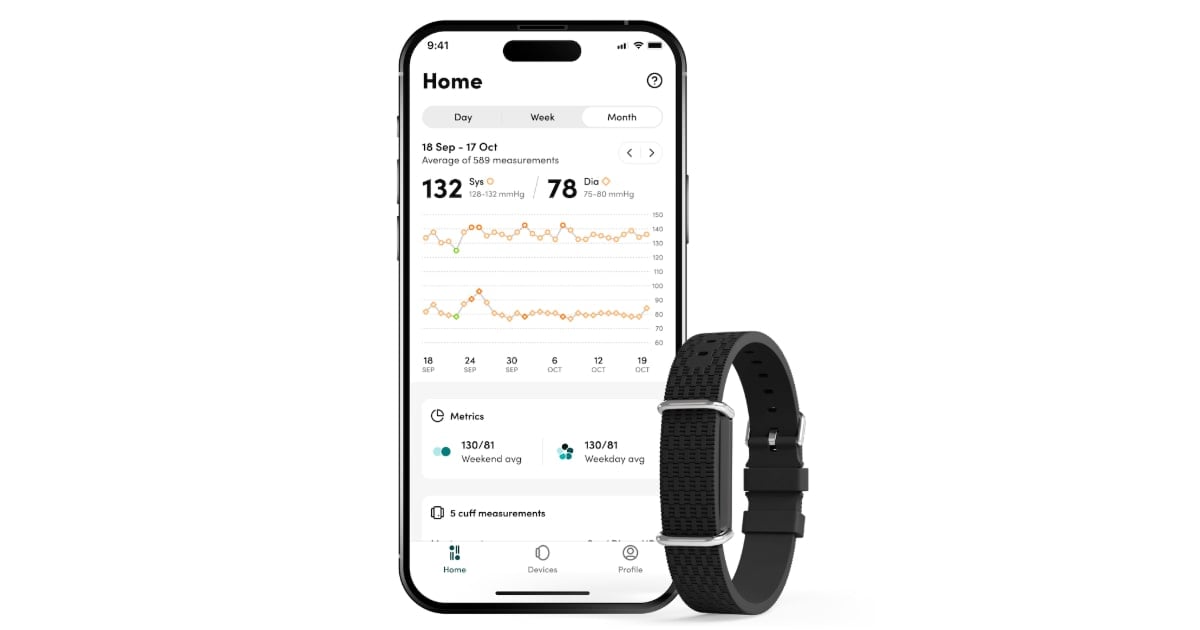
Aktiia’s blood pressure system, known under the Hilo brand in Europe, is a wearable that applys an optical sensor to collect data from one’s wrist. That data is then sent to the Hilo app and Hilo’s cloud server, where algorithms estimate the wearer’s blood pressure applying pulse wave analysis.
The company declares the analysis examines the unique form of pressure exerted by each heartbeat through the wearer’s blood vessels.
The wrist wearable continuously collects one’s blood pressure throughout the day, but does so only when they are still.
The product, which is already CE marked as a Class IIa medical device for monitoring blood pressure in adults in Europe, will be available to U.S. consumers in 2026.
“This milestone is deeply personal for us as founders and profoundly important for everyone at Aktiia. From day one, our mission has been to tackle the global hypertension crisis through technology. With this FDA clearance, we’re one step closer to realizing our vision: a future where healthy blood pressure is accessible to all,” Dr. Josep Solà, cofounder and chief technology officer at Aktiia, informed MobiHealthNews.
“What creates this moment truly historic is not just the clearance itself, but the fact that the FDA has approved the Aktiia device for over-the-counter apply, no prescription required. It’s a rare and demanding bar to meet, and a clear signal that our technology is not only clinically validated but ready for real-world, large-scale deployment.”
Dr. Mattia Bertschi, cofounder and CEO, informed MobiHealthNews that the FDA clearance supports the company expand its reach.
“This opens a new market for us, but more importantly, it confirms that Aktiia’s solution is safe, intuitive, and designed for the people who necessary it most. We believe this is just the launchning, and that cuffless, wearable, and over-the-counter blood pressure monitoring will soon become the global standard,” Bertschi declared.
“We’re working day and night to ensure that Hilo by Aktiia doesn’t just participate in this transformation… but leads it.”
THE LARGER TREND
In May, Aktiia scored $42 million in an oversubscribed Series B funding round, bringing its total raise to more than $100 million.
Last year, Aktiia completed a CHF 27 million ($30 million) funding round and received CE mark in Europe for its calibration-free technology CALFREE, which allows blood pressure data to be collected applying inputs from optical sensors commonly found in smartwatches and cameras of commercial smartphones.
The Swiss company declared that approval of its CALFREE tech would enable it to offer integration of its medical-grade optical blood pressure monitoring to third-party companies developing consumer devices, such as smartwatches, smartphone cameras, and smart bands.
In 2021, the company raised $17.5 million in Series A funding, and the year before, it secured $6.1 million (CHF 6 million) in funding.
The company, founded in 2018, was a spin-out of CSEM, a Swiss research and development center.















Leave a Reply